For sufferers with inflammatory bowel illness, antibiotics generally is a double-edged sword. The broad-spectrum medication typically prescribed for intestine flare-ups can kill useful microbes alongside dangerous ones, generally worsening signs over time. When preventing intestine irritation, you don’t at all times wish to deliver a sledgehammer to a knife struggle.
Researchers at MIT’s Pc Science and Synthetic Intelligence Laboratory (CSAIL) and McMaster College have identified a new compound that takes a extra focused strategy. The molecule, known as enterololin, suppresses a gaggle of micro organism linked to Crohn’s illness flare-ups whereas leaving the remainder of the microbiome largely intact. Utilizing a generative AI mannequin, the workforce mapped how the compound works, a course of that normally takes years however was accelerated right here to simply months.
“This discovery speaks to a central problem in antibiotic growth,” says Jon Stokes, senior creator of a new paper on the work, assistant professor of biochemistry and biomedical sciences at McMaster, and analysis affiliate at MIT’s Abdul Latif Jameel Clinic for Machine Studying in Well being. “The issue isn’t discovering molecules that kill micro organism in a dish — we’ve been in a position to try this for a very long time. A serious hurdle is determining what these molecules really do inside micro organism. With out that detailed understanding, you may’t develop these early-stage antibiotics into secure and efficient therapies for sufferers.”
Enterololin is a stride towards precision antibiotics: therapies designed to knock out solely the micro organism inflicting bother. In mouse fashions of Crohn’s-like irritation, the drug zeroed in on Escherichia coli, a gut-dwelling bacterium that may worsen flares, whereas leaving most different microbial residents untouched. Mice given enterololin recovered quicker and maintained a more healthy microbiome than these handled with vancomycin, a standard antibiotic.
Pinning down a drug’s mechanism of motion, the molecular goal it binds inside bacterial cells, usually requires years of painstaking experiments. Stokes’ lab found enterololin utilizing a high-throughput screening strategy, however figuring out its goal would have been the bottleneck. Right here, the workforce turned to DiffDock, a generative AI mannequin developed at CSAIL by MIT PhD pupil Gabriele Corso and MIT Professor Regina Barzilay.
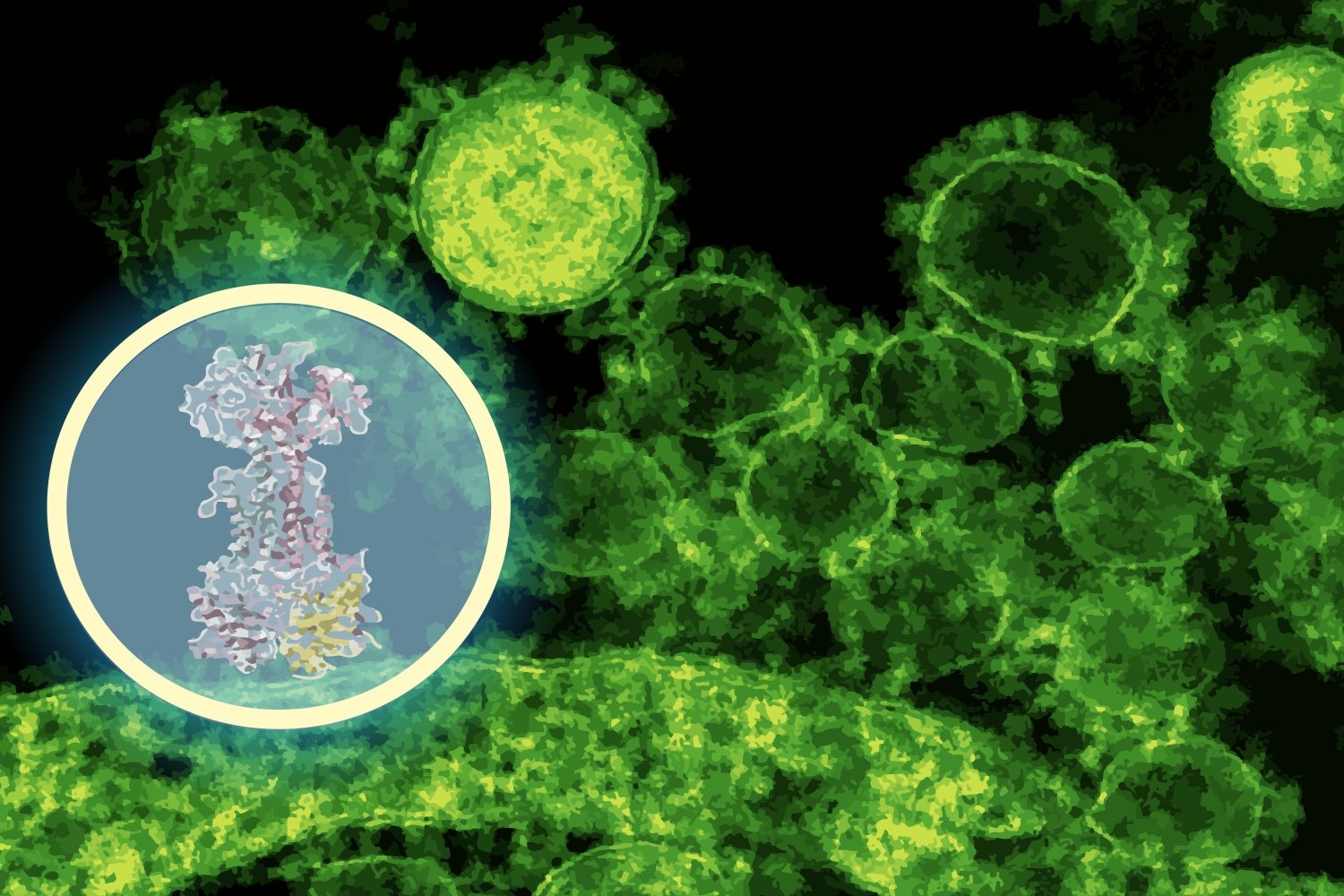
DiffDock was designed to foretell how small molecules match into the binding pockets of proteins, a notoriously troublesome downside in structural biology. Conventional docking algorithms search by way of potential orientations utilizing scoring guidelines, typically producing noisy outcomes. DiffDock as a substitute frames docking as a probabilistic reasoning downside: a diffusion mannequin iteratively refines guesses till it converges on the most probably binding mode.
“In simply a few minutes, the mannequin predicted that enterololin binds to a protein advanced known as LolCDE, which is important for transporting lipoproteins in sure micro organism,” says Barzilay, who additionally co-leads the Jameel Clinic. “That was a really concrete lead — one that might information experiments, relatively than change them.”
Stokes’ group then put that prediction to the check. Utilizing DiffDock predictions as an experimental GPS, they first advanced enterololin-resistant mutants of E. coli within the lab, which revealed that modifications within the mutant’s DNA mapped to lolCDE, exactly the place DiffDock had predicted enterololin to bind. In addition they carried out RNA sequencing to see which bacterial genes switched on or off when uncovered to the drug, in addition to used CRISPR to selectively knock down expression of the anticipated goal. These laboratory experiments all revealed disruptions in pathways tied to lipoprotein transport, precisely what DiffDock had predicted.
“Whenever you see the computational mannequin and the wet-lab information pointing to the identical mechanism, that’s whenever you begin to consider you’ve figured one thing out,” says Stokes.
For Barzilay, the venture highlights a shift in how AI is used within the life sciences. “Plenty of AI use in drug discovery has been about looking chemical house, figuring out new molecules that could be energetic,” she says. “What we’re exhibiting right here is that AI can even present mechanistic explanations, that are essential for transferring a molecule by way of the event pipeline.”
That distinction issues as a result of mechanism-of-action research are sometimes a serious rate-limiting step in drug growth. Conventional approaches can take 18 months to 2 years, or extra, and value thousands and thousands of {dollars}. On this case, the MIT–McMaster workforce reduce the timeline to about six months, at a fraction of the fee.
Enterololin remains to be within the early phases of growth, however translation is already underway. Stokes’ spinout firm, Stoked Bio, has licensed the compound and is optimizing its properties for potential human use. Early work can be exploring derivatives of the molecule in opposition to different resistant pathogens, corresponding to Klebsiella pneumoniae. If all goes nicely, medical trials may start inside the subsequent few years.
The researchers additionally see broader implications. Slim-spectrum antibiotics have lengthy been sought as a approach to deal with infections with out collateral injury to the microbiome, however they’ve been troublesome to find and validate. AI instruments like DiffDock may make that course of extra sensible, quickly enabling a brand new era of focused antimicrobials.
For sufferers with Crohn’s and different inflammatory bowel circumstances, the prospect of a drug that reduces signs with out destabilizing the microbiome may imply a significant enchancment in high quality of life. And within the larger image, precision antibiotics could assist deal with the rising menace of antimicrobial resistance.
“What excites me isn’t just this compound, however the concept that we will begin fascinated with the mechanism of motion elucidation as one thing we will do extra shortly, with the suitable mixture of AI, human instinct, and laboratory experiments,” says Stokes. “That has the potential to vary how we strategy drug discovery for a lot of illnesses, not simply Crohn’s.”
“One of many best challenges to our well being is the rise of antimicrobial-resistant micro organism that evade even our greatest antibiotics,” provides Yves Brun, professor on the College of Montreal and distinguished professor emeritus at Indiana College Bloomington, who wasn’t concerned within the paper. “AI is turning into an essential instrument in our struggle in opposition to these micro organism. This research makes use of a strong and chic mixture of AI strategies to find out the mechanism of motion of a brand new antibiotic candidate, an essential step in its potential growth as a therapeutic.”
Corso, Barzilay, and Stokes wrote the paper with McMaster researchers Denise B. Catacutan, Vian Tran, Jeremie Alexander, Yeganeh Yousefi, Megan Tu, Stewart McLellan, and Dominique Tertigas, and professors Jakob Magolan, Michael Surette, Eric Brown, and Brian Coombes. Their analysis was supported, partly, by the Weston Household Basis; the David Braley Centre for Antibiotic Discovery; the Canadian Institutes of Well being Analysis; the Pure Sciences and Engineering Analysis Council of Canada; M. and M. Heersink; Canadian Institutes for Well being Analysis; Ontario Graduate Scholarship Award; the Jameel Clinic; and the U.S. Protection Risk Discount Company Discovery of Medical Countermeasures Towards New and Rising Threats program.
The researchers posted sequencing information in public repositories and launched the DiffDock-L code brazenly on GitHub.


